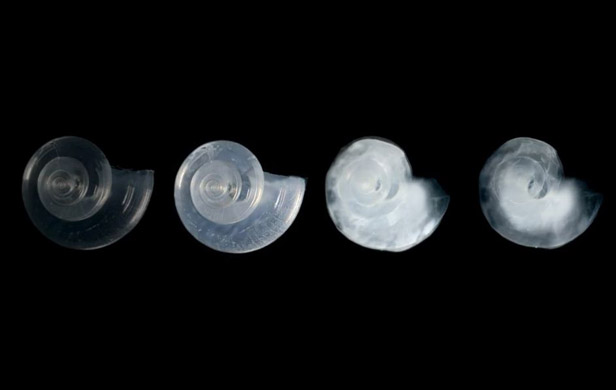
Research conducted at an ice camp high in the frozen North – part of the Catlin Arctic Survey – suggests climate change is threatening the Arctic Ocean’s food web by making those waters more acidic.
The scientists, who camped for months at a time on the sea ice near the magnetic North Pole, tested the effect of various acid levels on tiny, shrimp-like creatures called copepods (KOH-peh-pods) that almost all fish and whales depend on for food.
They found that some copepods do better than others in more acidic waters.
But their recently published research concludes that all the species they looked at suffered at some point in water with lower pH.
Carbon dioxide, the main gas responsible for climate change, increases the acidity of the oceans as it dissolves in seawater.
The Arctic Ocean is becoming more acidic at a faster pace than any other on Earth.
The paper was published in the journal Proceedings of the National Academy of Sciences (PNAS) and was carried out by the University of Exeter and the Plymouth Marine Laboratory.
Ocean “acidification” is just about the stupidest misreading of how the carbon cycle works.
Cool water holds more gas than warm water, which is why Northern waters are so much more productive than that in the tropics. CO2 feeds micro plant and animal life at the bottom of the food chain. The pH of ocean water depends on the time, place, and season the sample is taken.The ocean is still very alkaline, so “acidification” is just more flapdoodle.
The Current High-Frequency Dynamics of Ocean pH Reference
Hofmann, G.E., Smith, J.E., Johnson, K.S., Send, U., Levin, L.A., Micheli, F., Paytan, A., Price, N.N., Peterson, B., Takeshita, Y., Matson, P.G., Crook, E.D., Kroeker, K.J., Gambi, M.C., Rivest, E.B., Frieder, C.A., Yu, P.C. and Martz, T.R. 2011. High-frequency dynamics of ocean pH: A multi-ecosystem comparison. PLoS ONE 6: e28983.
Background
The authors write that “natural variability in pH is seldom considered when effects of ocean acidification are considered,” and they suggest that this omission is disturbing, because “natural variability may occur at rates much higher than the rate at which carbon dioxide is decreasing ocean pH,” which is about 0.0017 pH unit per year according to Dore et al. (2009) and Byrne et al. (2010). And because of this fact, they contend that “ambient fluctuation in pH may have a large impact on the development of resilience in marine populations,” noting that “heterogeneity in the environment with regard to pH and pCO2 exposure may result in populations that are acclimatized to variable pH or extremes in pH.”
What was done
To further explore this possibility, Hofmann et al. recorded continuous high-resolution time series of upper-ocean patterns of pH variability with autonomous sensors deployed at fifteen different locations stretching from 40.7303°N to 77.8000°S latitude and from 0 to 166.6712°E longitude and 0 to 162.1218°W longitude, over a variety of ecosystems ranging from polar to tropical, open-ocean to coastal, and kelp forest to coral reef.
What was learned
The eighteen researchers report that their measurements revealed “a continuum of month-long pH variability with standard deviations from 0.004 to 0.277 and ranges spanning 0.024 to 1.430 pH units,” which variability was “highly site-dependent, with characteristic diel, semi-diurnal, and stochastic patterns of varying amplitudes.”
What it means
Directly quoting Hofmann et al., “these biome-specific pH signatures disclose current levels of exposure to both high and low dissolved CO2, often demonstrating that resident organisms are already experiencing pH regimes that are not predicted until 2100.” And these facts suggest that the current real-world heterogeneity of the world’s oceans with regard to pH and pCO2 exposure may indeed “result in populations that are acclimatized to variable pH or extremes in pH,” such as those that have been predicted to be the new norm in 2100.
References
Byrne, R.H., Mecking, S., Feely, R.A. and Liu, X. 2010. Direct observations of basin-wide acidification of the North Pacific Ocean. Geophysical Research Letters 37: 10.1029/2009GL040999.
Dore, J.E., Lukas, R., Sadler, D.W., Church, M.J. and Karl, D.M. 2009. Physical and biogeochemical modulation of ocean acidification in the central North Pacific. Proceedings of the National Academy of Sciences USA 106: 12,235-12,240.
Reviewed 30 May 2012
http://co2science.org/articles/V15/N22/B3.php
Judy Cross, it is so exciting to read your words! That you cite a relevant paper, is even better. To the rest of you, Judy is correct – the eternally alkaline oceans are not threatened by carbon dioxide. The little critters studied near the north pole, however, might be more threatened by the erupting undersea volcanic ridge nearby. Did anyone else mention that? Not in this “ocean acidification” study. Take note, the researchers in the above article did not find acid ocean waters, no… they took samples of little critters, and subjected them to the acid baths which the researchers created… in their test-tubes (or other such containers) … not the ocean. The oceans will NEVER be acid. Never. The researchers discovered that some of the critters did better than other critters, but, with enough acid, they all croaked. What would you expect? The author (not the researchers) claims that CO2 is the problem, and also claims (the author, not the researchers) that the arctic is, well, here are the authors words:
“Carbon dioxide, the main gas responsible for climate change, increases the acidity of the oceans as it dissolves in seawater.
The Arctic Ocean is becoming more acidic at a faster pace than any other on Earth.”
It isn’t the researchers who said the Arctic Oceanis becoming more acidic, it is the author. Oh, and by the way, the terminology is just plain wrong. Shifting the pH of an alkaline solution (sea water) to a lower pH is just reducing the alkalinity, it is not proper to say the sea water is becoming “more acidic” … Those are the words of an activist, not a chemist.
The Arctic Ocean will NEVER be acidic. Never.
To the rest of you: The high school chemistry class you slept through should have taught you that certain chemicals (as they dissolve in water) will cause the pH of the water to resist changes…. we chemists call that “buffering” … and there are megatons of buffering chemicals in the ocean bottoms…. No, these aren’t chemicals dumped there by mankind, these are what y’all would call ROCKS. Yes, Rocks are chemicals. So the eternally alkaline oceans are buffered, and the pH of the oceans will not ever go into the acid range… if they do, mankind will not be around to measure the pH. Let me say that again, the pH of the oceans will always be alkaline, the oceans will never become acidic. Got that? Never. Now, the fresh water flowing into the oceans, from rivers, well, that water can pickup tannins, the tea-colored brown stuff, from dead plant matter. Tannins in fresh water can shift the pH into the acid range by forming tannic acid. There are other things that can make fresh water shift in pH. River water is no where near as buffered as the ever-alkaline oceans are. So, river water can flow into the ocean, and the river water can be acidic. Mixing and buffer reactions will eventually make this water alkaline, but until that happens, there can be acidic wafts flowing around. Also, the shallow waters over reefs can be locally overwhelmed by stuff (typically organic crap from live things) and it can become acidic. This will eventually be neutralized and shifted into the alkaline range by the eternally alkaline oceans, but locally, in shallow zones, waters can experience transient pH shifts. Transient – that means they don’t last long. The oceans will NEVER become acid. Never. They are too big, they are too buffered. Never. That doesn’t mean that some crap cannot mess things up, here and there. Crap happens, as Forrest observed. Not to worry…